

How can treatment help?
HRR gene-mutated mCRPC = prostate cancer with certain abnormal inherited or acquired genes called homologous recombination repair (HRR genes) that has spread to other parts of the body (metastatic) and no longer responds to a hormone therapy or surgical treatment to lower testosterone (castration-resistant).
In a study of patients with HRRm mCRPC, TALZENNA® (talazoparib) + XTANDI® (enzalutamide) reduced the risk of progression and helped patients live longer
The study
TALZENNA + XTANDI was studied in a clinical trial in adults with HRR gene-mutated mCRPC.
The main goal of the study was to measure progression-free survival, which means how long patients lived without their cancer getting worse or until they died for any reason. Another goal was overall survival, which is the time patients lived from the start of the study until death from any cause.
In the study, 200 patients were given TALZENNA + XTANDI, and 199 patients were given placebo + XTANDI. All patients either had surgery to lower testosterone or continued to take hormone therapy.


The results
Reduced risk of progression
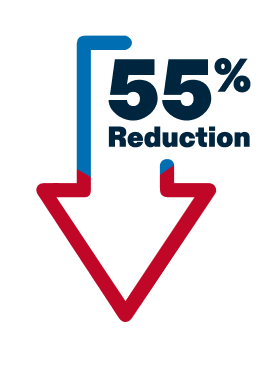
The risk of cancer progression was reduced by 55% with TALZENNA + XTANDI vs those taking placebo + XTANDI.
Cancer progression was seen in 66 of the 200 patients taking TALZENNA + XTANDI vs 104 of the 199 patients taking placebo + XTANDI.
The risk of cancer progression was reduced by 55% with TALZENNA + XTANDI vs those taking placebo + XTANDI.
Cancer progression was seen in 66 of the 200 patients taking TALZENNA + XTANDI vs 104 of the 199 patients taking placebo + XTANDI.
Median time until progression
The median length of time until cancer progression was not reached for TALZENNA + XTANDI.* In this clinical trial, patients taking TALZENNA + XTANDI had a 97.5% chance that the median time until cancer progression was at least 21.9 months.
The median length of time until cancer progression was 13.8 months for patients who took placebo + XTANDI.

*“Median not reached” means that more than half of patients were alive without their cancer progressing at the time of analysis.
TALZENNA + XTANDI helped patients live longer vs placebo + XTANDI

- Patients lived for a median of 3.8 years (45.1 months) with TALZENNA + XTANDI vs 2.6 years (31.1 months) with placebo + XTANDI. At the time of this analysis, 93 out of the 200 patients taking TALZENNA + XTANDI had died for any reason vs 126 out of the 199 patients taking placebo + XTANDI
- A median is the middle number in a group of numbers ordered from lowest to highest
- Median time until cancer progression is the length of time until half of the patients receiving treatment had their cancer progress (get worse) or until they died for any reason
- Median survival is the length of time after the start of the study that half of the patients were still alive
Results may vary. Ask your doctor if TALZENNA + XTANDI is right for you.
Talk to your doctor about TALZENNA + XTANDI
Our doctor discussion guide can help you ask more focused questions at your next appointment.

